Royal Melbourne Hospital: First Public Hospital in Australia to Adopt New Technology for Brain Tumour Surgery
Melbourne, Australia May 2012: Patients with an aggressive form of brain tumour, glioblastoma multiforme (GBM), have been given access to a new brain tumour visualisation drug, Gliolan® (5-aminolevulinic acid), at The Royal Melbourne Hospital.
In 2011, The Royal Melbourne Hospital trialled the drug with great success, and is expected to treat up to an additional 25 patients.
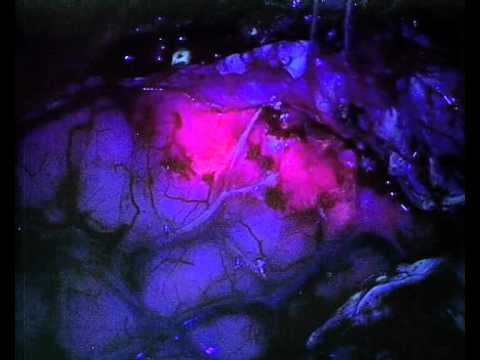
Gliolan causes brain tumours, known as gliomas, to become fluorescent during surgery. This enables neurosurgeons to better visualise these tumours and more completely remove them. Gliolan is given to the patient as a drink three hours before surgery. During surgery, a specially modified neurosurgical microscope fitted with a blue operating light is used, which causes cancerous tissue to glow fluorescent red whilst normal brain tissue appears blue.
Associate Professor Kate Drummond, a neurosurgeon at The Royal Melbourne Hospital, was the first surgeon in Victoria to use Gliolan.
“Gliolan has made a positive difference to how we can treat our patients with this aggressive form of brain cancer,” A/Professor Drummond said.
“We have found the drug to be a very useful tool during neurosurgery because it can highlight difficult to see pockets of brain tumour tissue.”
“This assists us to more thoroughly remove these difficult to treat brain tumours, providing patients with better outcomes.”
The Royal Melbourne Hospital was the first hospital in Australia to approve the use of Gliolan for patients with GBM. At present it is the only hospital in Victoria to offer this new treatment and it will now become a standard treatment for people with GBM which is able to be surgically removed.
International studies have shown that the use of Gliolan during surgery has nearly doubled the rate of achieving complete removal of the tumour, which has resulted in a doubling of the number of patients without progression of their brain cancer six months after their surgery.1
Gliolan is in-licensed by Melbourne biopharmaceutical company, Specialised Therapeutics Australia (STA).
STA chief executive officer Mr Carlo Montagner said: “Neurosurgeons at The Royal Melbourne Hospital can now use Gliolan to assist them when performing extremely complex brain surgery. Our ultimate aim is for Gliolan to become widely available in hospitals right around Australia to improve outcomes for all GBM patients.”
Gliolan is not yet approved but has been granted orphan drug designation by the Therapeutic Goods Administration (TGA). STA will be filing for regulatory approval later this year. Prior to TGA approval, Gliolan is being made available to Australian neurosurgeons through the federal government’s Special Access Scheme.
References:
- Stummer W, Pichlmeier U, Meinel T, et al., Fluorescence-guided surgery with 5-aminovulinec acid for resection of malignant glioma: a randomised controlled multicentre phase III trial, Lancet Oncol, 2006;7:392-401
About Gliolan®
The active substance in Gliolan is 5-aminolevulinic acid. It is absorbed by cells in the body, where it is converted by enzymes into fluorescent chemicals, particularly protoporphyrin IX (PPIX). Since glioma cells take up more of the active substance and convert it more rapidly into PPIX, higher levels of PPIX accumulate in the cancer cells than in normal tissue. When illuminated under blue light of a specific wavelength, the PPIX in the tumour glows an intense red, while the normal brain tissue appears blue. This enables the surgeon to see the tumour more clearly during brain surgery and to remove it more accurately, sparing healthy brain tissue.
Like all medications Gliolan may cause side effects. Gliolan should not be used in patients with hypersensitivity to 5-ALA or porphyrins, in cases of acute or chronic porphyria, or in pregnancy. Cardiac disorders, gastrointestinal disorders and skin and subcutaneous disorders are all reported as being uncommon.
Gliolan is under license from photonamic GmbH and Co. KG.
- The Royal Melbourne Hospital to treat more patients with brain tumour drug Gliolan®
- Twice as many patients are without progression of their brain cancer six months after surgery with Gliolan
About Specialised Therapeutics Australia, Pty Ltd
Specialised Therapeutics Australia Pty Ltd (STA) was established to identify, develop and commercialise innovative anti-cancer and other specialised therapies for the Australasian market. Currently STA markets two world leading cancer and cancer supportive care therapies, ABRAXANE® (nanoparticle albumin-bound paclitaxel) and ALOXI® (palonosetron) respectively. Based in Melbourne, Australia, the privately held company is currently negotiating the rights to several more important therapeutic agents for release in Australasia and other regional markets.